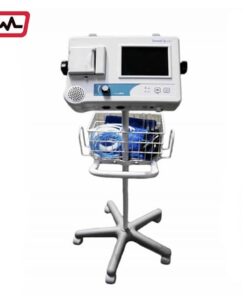
Summit Doppler VANMAX Vantage ABI
NEW
Patient Ready
Warranty Included

Description
Summit Doppler VANMAX Vantage ABI
Summit Doppler VANMAX Vantage ABI is a simple One-touch ABI Screening system in just 3 minutes.
The true one-button ABI system that performs the ABI exam in as little as three minutes. Now, diagnosing Peripheral Arterial Disease for the 8-12 million Americans affected can be done fast and accurately.
The Vantage ABI uses an innovative cuff-based technology for performing the ankle-brachial index (ABI) exam to assist in the diagnosis of peripheral arterial disease (P.A.D.).
The Summit Doppler VANMAX Vantage ABI provides accurate determination of the ABI across the full range of P.A.D. The technology, developed by Summit Doppler, was co-invented in cooperation with researchers from the Cleveland Clinic. It uses DFO waveform analysis, a significant improvement in ankle pressure determination and peripheral arterial disease assessment over traditional forms of oscillometric ankle pressure estimation.
The Vantage ABI conducts fast, accurate ABI screenings in minutes to assist with the early detection of Peripheral Arterial Disease (P.A.D.). The Vantage ABI completes a single level exam using Digital Fourier Oscillometry (DFO). Summit Doppler worked with researchers from the Cleveland Clinic to create a clinically validated unique DFO Algorithm*.
Vantage ABI System features include:
Speedy throughput with large display and intuitive touchscreen navigation
Convenient USB drive for data storage, transfer, and EMR interface
Blood pressure cuffs for obtaining pressures and ankle waveforms
One-touch three-minute ABI exams with USB data storage
Cuffs
Built-in printer
Basket
Durable stand
Vantage ABI Specifications:
Main Unit Dimensions– 6.4 in. x 11 in. x 7.3 in.
Main Unit Weight– 4.6 lbs
Mounting– Self-supported (rubber feet) or stand mounted (optional)
Portability– Built-in carry handle
Cuff Pressure Range– 0 to 280 mmHg
Pressure Gage Accuracy– +/–3 mmHg 0–200 mmHg, +/–2% >200 mmHg
Deflation Rate– 2.5 mmHg/second nominal
Inflation Pressure– 180 mmHg (default), 280 mmHg (user selected)
Deflation Pressure– 60 mmHg (default), 35 mmHg (user selected)
Pulse Rate Range– 43 to 120 BPM
ABI Determination Time– 3 minutes
Determination– Pulse Volume Oscillometry
Waveform Type– Pnuematic plethysmography (Pulse Volume Recording PVR)
Patient Connections– 4 vascular cuffs, 6 ft. hose length (ea.)
Clinical Sensitivity– >90%
Clinical Specificity– >92%
Standards– IEC60601-1, IEC60601-1-2 EMC
Classification– Class A
Hose/Cable Length– 6 ft.
Dimensions:
- Depth: 11 in/27.9 cm
- Width: 7.3 in/18.5 cm
- Height: 6.4 in/16.25 cm
- Weight: 4.6 lbs/2.1 Kg
- Mounting: Self‐supported (rubber feet) or stand mounted (optional)
- Portability: Built‐in carry handle
Clinical: - Cuff Pressure Range: 0 to 280 mmHg
- Pressure Gage Accuracy: +/-3 mmHg 0‐200 mmHg, +/-2% >200 mmHg
- Deflation Rate: 2.5 mmHg/second nominal
- Inflation Pressure: 180 mmHg (Default); 280 mmHg (User selected)
- Deflation Pressure: 60 mmHg (Default); 35 mmHg (User selected)
- Pulse Rate Range: 43 to 120 BPM
- ABI Determination Time: 3 minutes
- Determination: Pulse Volume Oscillometry – Patent Pending
- Waveform Type: Pnuematic Plethysmography ( Pulse Volume Recording ‐PVR)
- Patient Connections: 4 Vascular Cuffs, 6 foot hose length (ea.)
- Clinical Sensitivity: > 90%
- Clinical Specificity: > 92%
Battery: - Type: 7.2V NiMH (custom pack with thermal and over‐current protection)
- Replacement Part: B200
- Replacement Interval: 1 year
External Power Adaptor: - Input: 100-240VAC 50/60 Hz 1.2 Amp
- Output: + 7VDC, 5A
Environmental: - Operating Temperature: 10 – 30° C
- Operating Humidity: 30 – 75% non‐condensing
- Transport/Storage Temperature: [-20] to 50° C
- Transport/Storage Humidity: 5 – 90% non‐condensing